 | |
 | |
| Names | |
|---|---|
| IUPAC name
5ξ-Pregnane[1] | |
| Systematic IUPAC name
(1S,3aS,3bS,5aΞ,9aS,9bS,11aR)-1-Ethyl-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H36 | |
| Molar mass | 288.511 g/mol |
| Density | 0.926 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
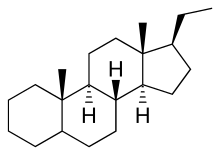
Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane (originally allopregnane) and 5β-pregnane (17β-ethyletiocholane). It has a gonane core.
5β-Pregnane is the parent of pregnanediones, pregnanolones, and pregnanediols, and is found largely in urine as a metabolic product of 5β-pregnane compounds.
Pregnanes
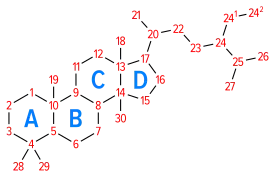
Pregnanes are steroid derivatives with carbons present at positions 1 through 21.
Most biologically significant pregnane derivatives fall into one of two groups: pregnenes and pregnadienes. Another class is pregnatrienes.
Pregnenes
Pregnenes have a double bond. Examples include:
Pregnadienes
Pregnadienes have two double bonds. Examples include:
See also
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1530. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
External links
 Media related to Pregnane at Wikimedia Commons
Media related to Pregnane at Wikimedia Commons- Pregnanes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- PubChem
- Diagram at qmul.ac.uk
- Definition of Pregnane
- Progesterone Chemistry
- Progesterone record in European Bioinformatics database