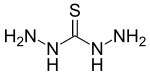
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hydrazinecarbothiohydrazide[1] | |
| Other names
1,3-Diamino-2-thiourea; Thiocarbazide; Thiocarbonic dihydrazide; Thiocarbonyldihydrazide; Carbonothioic dihydrazide; TCh; Thiocarbonohydrazide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.017.064 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH6N4S | |
| Molar mass | 106.15 g·mol−1 |
| Melting point | 171 to 174 °C (340 to 345 °F; 444 to 447 K) (decomposes)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Thiocarbohydrazide is a toxic compound made by the reaction of carbon disulfide with hydrazine (hydrazinolysis). It is used in the silver proteinate specific staining of carbohydrates in electron microscopy.
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 878. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Thiocarbohydrazide at Sigma-Aldrich
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.