 | |
| Names | |
|---|---|
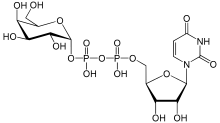
| IUPAC name
Uridine 5′-(α-D-galactopyranosyl dihydrogen diphosphate) | |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxyoxolan-2-yl]methyl} O3-[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Uridine+diphosphate+galactose |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H24N2O17P2 | |
| Molar mass | 566.302 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uridine diphosphate galactose (UDP-galactose) is an intermediate in the production of polysaccharides.[1] It is important in nucleotide sugars metabolism, and is the substrate for the transferase B4GALT5.
Sugar Metabolism
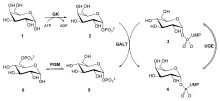
UDP-Galactose is especially relevant in glycolysis. It is derived from galactose an epimer of glucose, and via the Leloir Pathway, it is used be used as a precursor for the metabolism of glucose into pyruvate.[2] When lactose is hydrolyzed, D-Galactose enters the liver via the bloodstream. There, galactokinase phosphorylates it to galactose-1-phosphate using ATP. This compound then engages in a "ping-pong" reaction with UDP-Glucose, catalyzed by uridylyltransferase, yielding glucose-1-phosphate and UDP-Galactose. This glucose-1-phosphate feeds into glycolysis, while UDP-Galactose undergoes epimerization to regenerate UDP-Glucose.[3]
See also
References
- ↑ "Galactose 1 Phosphate Uridyltransferase Deficiency". StatPearls. StatPearls. 2022.
- ↑ Garrett, Reginald H.; Grisham, Charles M. (2017). Biochemistry (6th ed.). Boston, MA, USA: Cengage Learning. ISBN 978-1-305-57720-6.
- ↑ Nelson, David L.; Cox, Michael M.; Nelson, David L. (2013). Lehninger, Albert L. (ed.). Lehninger principles of biochemistry (6th ed.). Basingstoke: Macmillan Higher Education. ISBN 978-1-4292-3414-6.