 | |
| Names | |
|---|---|
| Other names
2,6,6-trimethylbicyclo[3.1.1]heptan-2-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.789 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.253 g·mol−1 |
| Appearance | colorless solid |
| Melting point | 78–79 °C (cis) 58–59 °C (trans |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Danger | |
| H302, H311, H312, H315, H319 | |
| P264, P264+P265, P270, P280, P301+P317, P302+P352, P305+P351+P338, P316, P317, P321, P330, P332+P317, P337+P317, P361+P364, P362+P364, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
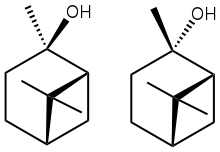
2-Pinanol is a collection of bicyclic terpenoid derived from the terpene pinene, but containing a tertiary alcohol. Both cis and trans isomers exist. Both are chiral They are produced by deoxygenation of corresponding cis- and trans-2-pinane hydroperoxide, which in turn can be produced by autoxidation of pinane with air.[2] Heating 2-pinanol gives linalool.[3]
References
- ↑ "2-Pinanol". pubchem.ncbi.nlm.nih.gov.
- ↑ Erman, Mark B.; Kane, Bernard J. (2008). "Chemistry Around Pinene and Pinane: A Facile Synthesis of Cyclobutanes and Oxatricyclo-Derivative of Pinane from cis- and trans-Pinanols". Chemistry & Biodiversity. 5 (6): 910–919. doi:10.1002/cbdv.200890104. PMID 18618388. S2CID 24782774.
- ↑ Eggersdorfer, Manfred (2000). "Terpenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_205. ISBN 978-3527306732.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.