 | |
| Names | |
|---|---|
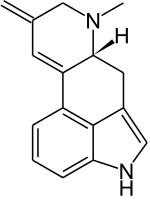
| IUPAC name
6-Methyl-8-methylidene-9,10-didehydroergoline | |
| Systematic IUPAC name
(6aR)-7-Methyl-9-methylidene-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline | |
| Other names
Lysergen; 9,10-Didehydro-6-methyl-8-methylene-ergoline | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H16N2 | |
| Molar mass | 236.318 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lysergene is an ergot alkaloid.[1]
References
- ↑ Pertz, H (1996). "Naturally occurring clavines: Antagonism/partial agonism at 5-HT2A receptors and antagonism at alpha 1-adrenoceptors in blood vessels". Planta Medica. 62 (5): 387–92. doi:10.1055/s-2006-957922. PMID 8923801.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.