 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.756 |
| Chemical and physical data | |
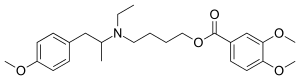
| Formula | C25H35NO5 |
| Molar mass | 429.557 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Mebeverine is a drug used to alleviate some of the symptoms of irritable bowel syndrome. It works by relaxing the muscles in and around the gut.[1]
Medical use
Mebeverine is used to alleviate some of the symptoms of irritable bowel syndrome (IBS) and related conditions; specifically stomach pain and cramps, persistent diarrhoea, and flatulence.[2]
Data from controlled clinical trials have not found a difference from placebo or statistically significant results in the global improvement of IBS.[3][4]
It has not been tested in pregnant women nor in pregnant animals so pregnant women should not take it; it is expressed at low levels in breast milk, while no adverse effects have been reported in infants, breastfeeding women should not take this drug.[1]
Adverse effects
Adverse effects include hypersensitivity reactions and allergic reactions, immune system disorders, skin disorders including hives, oedema and widespread rashes.[2]
Additionally, the following adverse effects have been reported: heartburn, indigestion, tiredness, diarrhoea, constipation, loss of appetite, general malaise, dizziness, insomnia, headache, and decreased pulse rate.[1]
It does not have systemic anticholinergic side effects.[2]
Mebeverine can, on highly rare occasions, cause drug-induced acute angle closure glaucoma.[5]
In a urine drug-screening test, mebeverine can affect a false positive result for amphetamines.[6]
Mechanism of action
Mebeverine is an anticholinergic but its mechanism of action is not known; it appears to work directly on smooth muscle within the gastrointestinal tract and may have an anaesthetic effect, may affect calcium channels, and may affect muscarinic receptors.[2]
It is metabolized mostly by esterases, and almost completely. The metabolites are excreted in urine.[2]
Mebeverine exists in two enantiomeric forms. The commercially available product is a racemic mixture of them. A study in rats indicates that the two have different pharmacokinetic profiles.[7]
History
It is a second generation papaverine analog, and was first synthesized around the same time as verapamil.[8]
It was first registered in 1965.[9]
Availability
Mebeverine is a generic drug and is available internationally under many brand names.[10]
References
- 1 2 3 "Colofac data sheet" (PDF). New Zealand Medicines and Medical Devices Safety Authority. 14 June 2017. Retrieved 21 July 2017.
- 1 2 3 4 5 "Colofac Tablets 135mg - Summary of Product Characteristics (SPC)". UK Electronic Medicines Compendium. 26 August 2016. Retrieved 21 July 2017.
- ↑ Annaházi A, Róka R, Rosztóczy A, Wittmann T (May 2014). "Role of antispasmodics in the treatment of irritable bowel syndrome". World Journal of Gastroenterology. 20 (20): 6031–43. doi:10.3748/wjg.v20.i20.6031. PMC 4033443. PMID 24876726.
- ↑ Darvish-Damavandi M, Nikfar S, Abdollahi M (February 2010). "A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome". World Journal of Gastroenterology. 16 (5): 547–53. doi:10.3748/wjg.v16.i5.547. PMC 2816265. PMID 20128021.
- ↑ Lachkar Y, Bouassida W (March 2007). "Drug-induced acute angle closure glaucoma". Current Opinion in Ophthalmology. 18 (2): 129–33. doi:10.1097/ICU.0b013e32808738d5. PMID 17301614. S2CID 30903966.
- ↑ Optimisation, NECS Medicines (2015-07-21). "Misuse of hyoscine butylbromide (Buscopan) |". medicines.necsu.nhs.uk. Retrieved 2018-02-07.
- ↑ Hatami M, Farhadi K, Tukmechi A (August 2012). "Fiber-based liquid-phase micro-extraction of mebeverine enantiomers followed by chiral high-performance liquid chromatography analysis and its application to pharmacokinetics study in rat plasma". Chirality. 24 (8): 634–9. doi:10.1002/chir.22057. PMID 22700279.
- ↑ Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 132. ISBN 9780471899792.
- ↑ "Mebeverine". druginfosys. Retrieved 1 February 2015.
- ↑ "Mebeverine". International. drugs.com. Retrieved 1 February 2015.