 | |
| Names | |
|---|---|
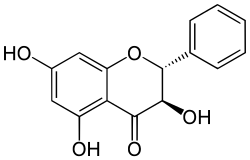
| IUPAC name
(2R,3R)-3,5,7-Trihydroxyflavan-4-one | |
| Systematic IUPAC name
(2R,3R)-3,5,7-Trihydroxy-2-phenyl-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
3,5,7-trihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12O5 | |
| Molar mass | 272.25 g/mol |
| Density | 1.497 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pinobanksin is an antioxidant bioflavonoid (specifically a flavanonol, a category of flavonol) that inhibits peroxidation of low density lipoprotein and it has electron donor properties reducing alpha-tocopherol radicals. It is present in sunflower honey.[1]
Pinobanksin is biosynthesized from pinocembrin.
References
- ↑ Sabatier, S.; Amiot, M.J.; Tacchini, M.; Aubert, S. (1992). "Identification of Flavonoids in Sunflower Honey". Journal of Food Science. 57 (3): 773. doi:10.1111/j.1365-2621.1992.tb08094.x.
External links
 Media related to Pinobanksin at Wikimedia Commons
Media related to Pinobanksin at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.