 | |
| Names | |
|---|---|
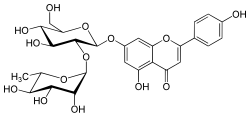
| IUPAC name
4′,5-Dihydroxy-7-[α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosyloxy]flavone | |
| Systematic IUPAC name
7-{[(2S,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-2-yl]oxy}-5-hydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.037.562 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H30O14 | |
| Molar mass | 578.52 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Rhoifolin is a chemical compound. It is first isolated from plant Rhus succedanea. The term "Rhoi" derived from generic name of plant Rhus.[1] It is a flavone, a type of flavonoid isolated from Boehmeria nivea, China grass or ramie (leaf), from Citrus limon, Canton lemon (leaf), from Citrus x aurantium, the bigarade or bitter orange (plant), from Citrus x paradisi, the grapefruit (leaf), from Ononis campestris, the cammock (shoot) and from Sabal serratula, the serenoa or sabal fruit (plant).[2]
References
- ↑ Hattori, Shizuo; Matsuda, Hiroaki (May 1952). "Rhoifolin, a new flavone glycoside, isolated from the leaves of Rhus succedanea". Archives of Biochemistry and Biophysics. 37 (1): 85–89. doi:10.1016/0003-9861(52)90164-1.
- ↑ Rhoifolin on Liber Herbarum Minor
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.