 | |
| Names | |
|---|---|
| IUPAC name
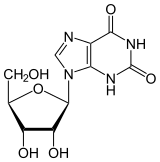
Xanthosine[2] | |
| Systematic IUPAC name
9-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3,9-dihydro-1H-purine-2,6-dione | |
| Other names
Xanthine riboside; 9-β-D-Ribofuranosylxanthine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.164 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C10H12N4O6 | |
| Molar mass | 284.228 g·mol−1 |
| Melting point | Decomposes when heated |
| Sparingly soluble in cold water; freely soluble in hot water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Xanthosine is a nucleoside derived from xanthine and ribose. It is the biosynthetic precursor to 7-methylxanthosine by the action of 7-methylxanthosine synthase. 7-Methylxanthosine in turn is the precursor to theobromine (active alkaloid in chocolate), which in turn is the precursor to caffeine, the alkaloid in coffee and tea.[3]
See also
- Xanthosine monophosphate
- Xanthosine diphosphate
- Xanthosine triphosphate
References
- ↑ Merck Index, 11th Edition, 9974
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1421. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Ashihara, Hiroshi; Yokota, Takao; Crozier, Alan (2013). "Biosynthesis and catabolism of purine alkaloids". Advances in Botanical Research. 68: 111–138. doi:10.1016/B978-0-12-408061-4.00004-3. ISBN 9780124080614.
{{cite journal}}: Cite journal requires|journal=(help)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.